Adapting your study data to evolving standards presents numerous considerations in order to ensure your study data is submission ready. The Standard for Exchange of Nonclinical Data (SEND), is an implementation of the SDTM standard for nonclinical studies and is a prime example of updated standards that are crucial to be considerate of. SEND was developed by the Clinical Data Interchange Standard Consortium (CDISC) and provides a standardized format for the submission of nonclinical Data to regulatory authorities such as the FDA(FDA, Providing Regulatory Submissions In Electronic Format — Standardized Study Data Guidance for Industry, June 2021).
A complete SEND package is the combination of three major components:
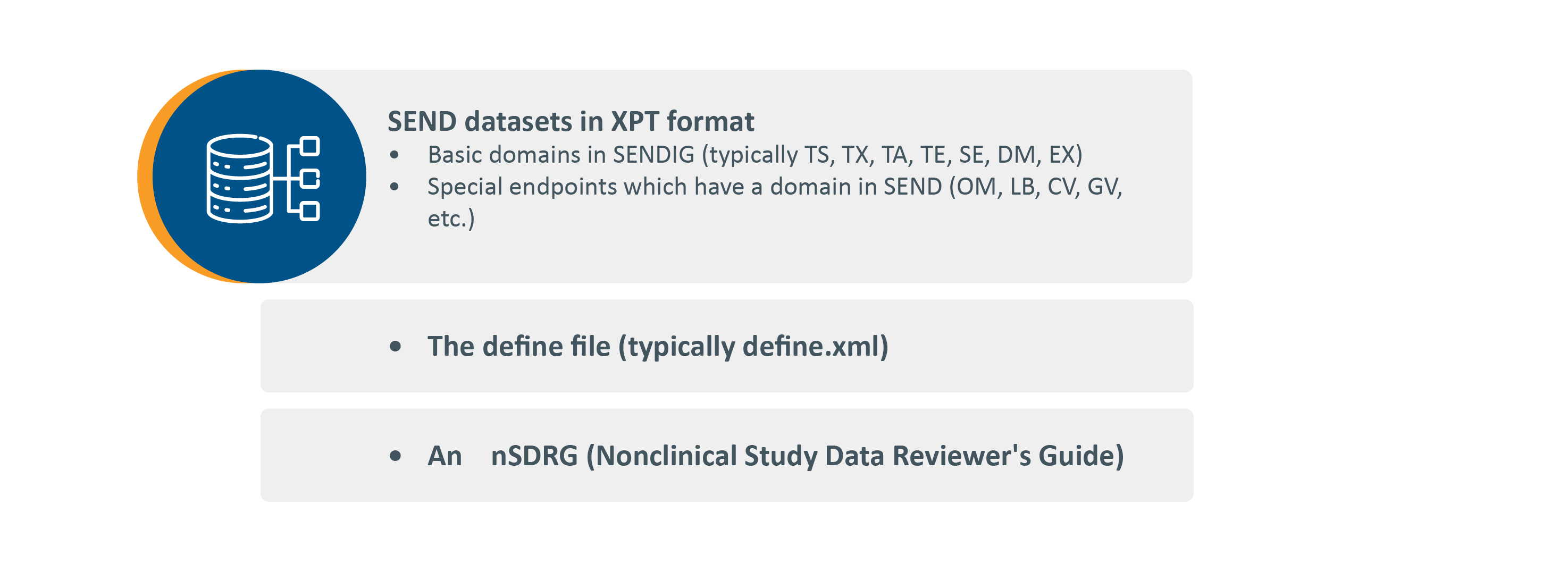
Domain Key:
- TS – Trial Summary
- TX – Trial Sets
- TA – Trial Arms
- TE – Trial Elements
- OM – Organ Measurements
- SE – Subject Elements
- DM – Demographics
- EX – Exposure
- LB – Laboratory Test Results
- CV – Cardiovascular Test Results
- GV – Genetic Toxicology – In Vivo
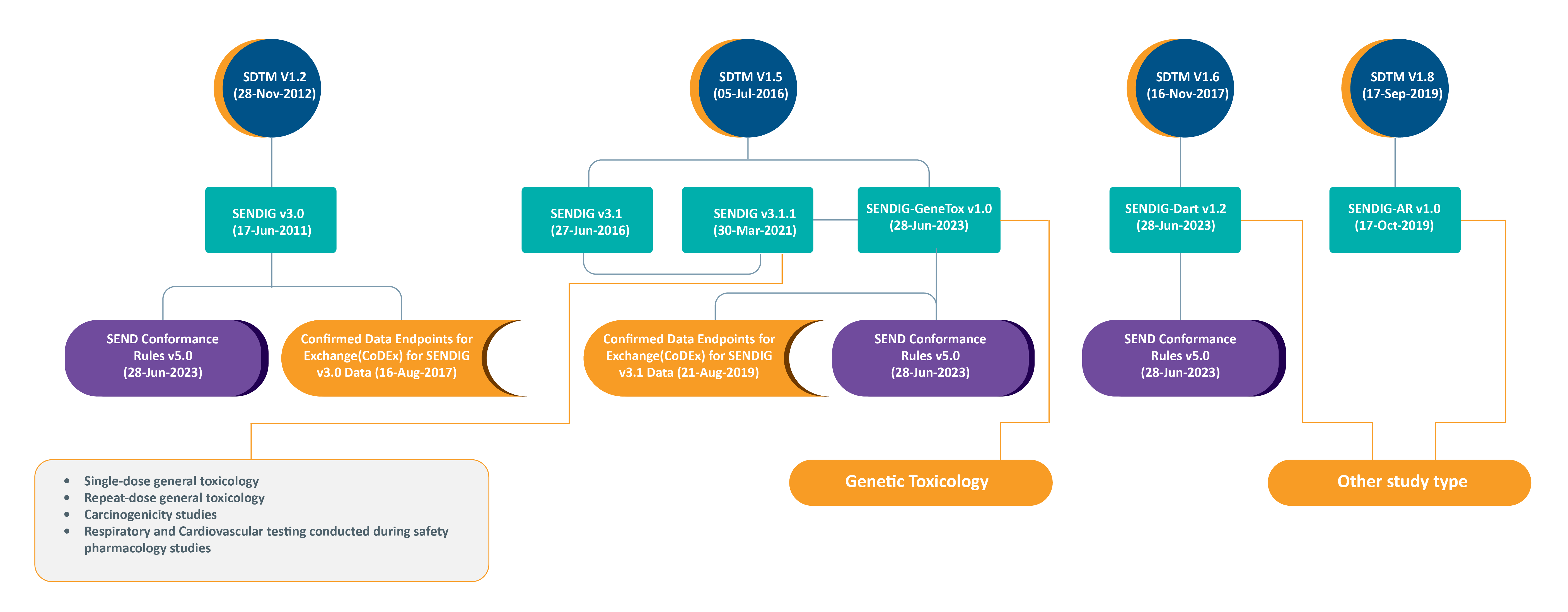
SENDIG (Standard for Exchange of Nonclinical Data Information Guide) Versions
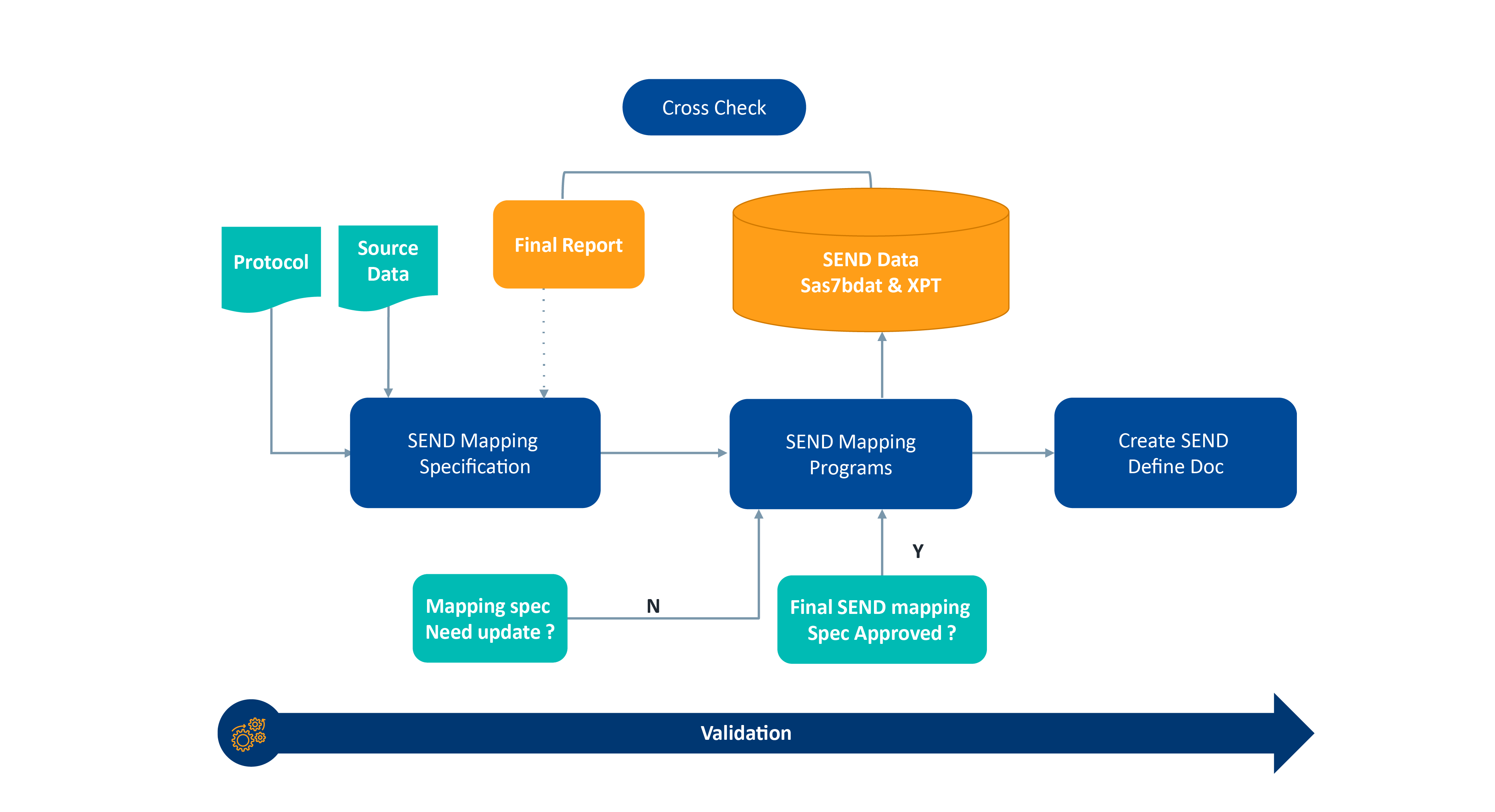
SEND Mapping Process Flow Chart
What are the Challenges of Preparing for Clinical Data Submissions?
While there are many factors to consider when preparing data for submissions, there are three major challenges to be aware of: source data, time requirements, and cross-referencing the final report with SEND.
Source Data
A significant challenge to expect is collecting source data in an organized and readily accessible format. For example, when working with lab data, data scientists/analysts manually transfer handwritten lab data from paper or PDFs into an electronic format. This process can introduce potential ambiguities and often incurs additional time and costs given the need for further quality control verification.
Time Requirements
Adjusting timelines to the requirements of SEND implementation is another challenge many are trying to navigate. With updates to SEND requirements, and more expected in the coming years, some remain unaware of the additional time required until the last minute. This raises concern as timely data transfer from each vendor is essential. These factors can delay submission timelines and often lead to an unanticipated increase in cost.
Cross-referencing the Final Report with SEND
Lastly, comparing the final report to the SEND datasets and documenting the discrepancies in nSDRG (Nonclinical Study Data Reviewer’s Guide) can create unexpected issues. In some instances, it may not be possible to represent a collected data element described in the study report as a standardized data element. In these cases, the nSDRG should provide an explanation for why certain data elements could not be fully standardized or were otherwise not included in the standardized data submission. This process adds further steps that may affect timelines.
Our Approach
Accelerating Source Data Collection
We engage early on with vendors and maintain clear and precise communication to establish the data transfer format. This ensures that all parties are aligned on the format and standards required for the SEND package.
Our experts leverage a proactive approach rather than reacting. Solving issues before they occur prevents unnecessary consequences to timelines and budget. Our team is educated and highly experienced in processes for early identification which allows for timely corrections, minimizing delays and ensuring data quality. By addressing potential issues at an early stage, we can prevent problems from escalating later in the process.
Overcoming Time Restrictions
Over the course of our 30 years of experience, we have become well-equipped to recognize the hurdles that come with adapting to new requirements and approach each project with a commitment to deliver a high-quality data package on time. Our experience enables us to engage with clients and their vendors while educating all parties on SEND submissions. This ensures that we adhere to specified timelines and budgets, helping to avoid delays and unexpected costs.
Ensuring Consistencies Between the Final Report with SEND
We prioritize cross-checking and solidifying comparisons between the final report and the SEND datasets as soon as we receive the draft report. Should any questions or discrepancies arise, by staying ahead of the curve, we can promptly report any considerations to the client or the vendor responsible for the final report early on. This proactive approach ensures that issues are addressed quickly and enhances accuracy. Furthermore, we use an internal SEND package checklist to validate both the data package and the final report. This ensures that any unresolved discrepancies are documented in nSDRG which fuels transparency and traceability.
Why Partner with ClinChoice for Your Submission?
ClinChoice understands the nuances that accompany implementation updates as it relates to SEND. In understanding the hurdles that accompany adapting to new requirements, we approach each project with guaranteed precise data and meticulous analysis which ensures traceability and transparency. In turn, we are able to adhere to your specified timelines and budget. ClinChoice is also an early adopter and ongoing contributor to CDISC standard development. We ensure your data is fully validated and submission ready. By working closely with CDISC, our team of experts can implement updates and adaptations to standards seamlessly within your study data.
About ClinChoice
ClinChoice is a global full-service CRO specializing in clinical development and functional
solutions for pharmaceutical, biotechnology, medical device, and consumer health
companies. We have 30 years of proven high-quality delivery and results across all
our services. With over 4,000 professionals in more than 20 countries across the Americas,
Europe, and Asia-Pacific, we are positioned to fulfill our clients’ business requirements
locally and globally. We offer high-quality, full-service clinical development and postmarketing
solutions. For our clients, it means a reliable partner and quality results.
References
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/providing-regulatory-submissions-electronic-format-standardized-study-data
- https://www.cdisc.org/standards/foundational/send
