The world is progressing rapidly with advanced technologies and customization in almost every sector to meet human needs. In the healthcare sector, manufacturers of medical devices are actively involved in developing individualized products based on a patient’s needs. In some instances, over-the-counter/mass-produced medical devices, especially implantable devices, may not meet the specific patient’s requirements. Hence, custom-made devices (CMDs) are required to fulfill such individual patients’ healthcare needs.
This article summarizes current medical device regulatory requirements for CMDs in the European Union (EU), and how these requirements differ from other medical devices.
What are Custom-made medical devices devices?
The EU Medical Device Regulations (MDR) Article 2(3) defines a ‘custom-made device’ as “any device that is specifically made following a written prescription of any person authorized by national law by virtue of that person’s professional qualifications, which gives, under that person’s responsibility, specific design characteristics, and is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.”
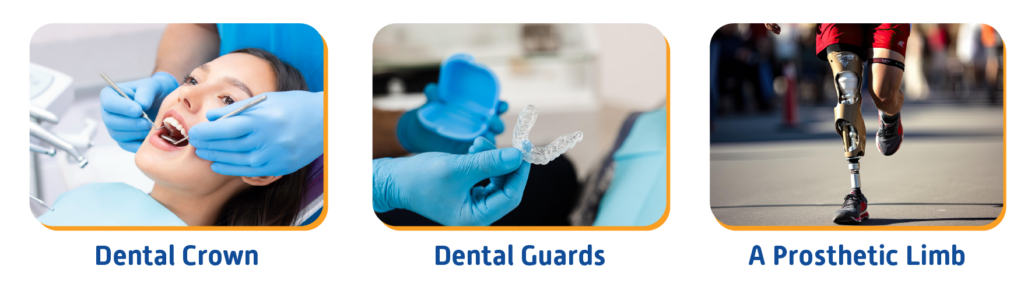
Simply put, CMD is a medical device designed for a specific patient to suit their anatomical and physiological needs as prescribed by a legally authorized and qualified professional who provides specific design characteristics under their responsibility. For example, dental crowns/patient-specific dental guards, and prostheses are all CMDs, as pictured below (Figure 1).
Figure 1: Examples of Custom-made devices
However, not all medical devices tailored to meet an individual patient/user’s needs following an individualized written prescription by a qualified health professional are classified as CMDs.
Personalized medical devices are often misconstrued as CMDs. There are two types of personalized medical devices: adaptable medical devices (such as spectacle frames and optical glasses) and patient-matched devices (such as orthopedic plates, mandibular plates, and externally worn orthoses). Even though these devices are intended to suit an individual’s needs, they are primarily mass-produced utilizing industrial manufacturing procedures to meet specific basic requirements, which are then further tailored to meet an individual patient/user’s need.
In other words, a medical device based on standardized dimensions or designs not designed only for a particular individual and typically produced in a continuous production run or homogenous batch cannot be categorized as a CMD.
How are the requirements for Custom-made devices different from other medical devices?
Custom-made device manufacturers must comply with EU MDR before placing their products on the European market to ensure safety and performance to meet design standards. However, unlike other medical device manufacturers, CMD manufacturers must meet only specific regulations. Table 1 provides an overview of these specific MDR requirements applicable to CMDs compared to other medical devices.
Table 1: Regulatory requirements of Custom-made and other medical devices
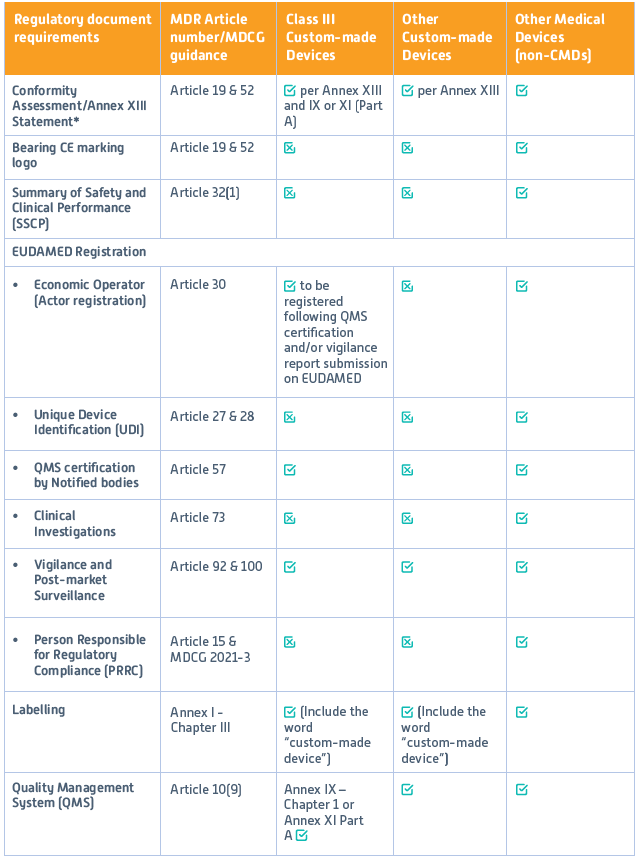
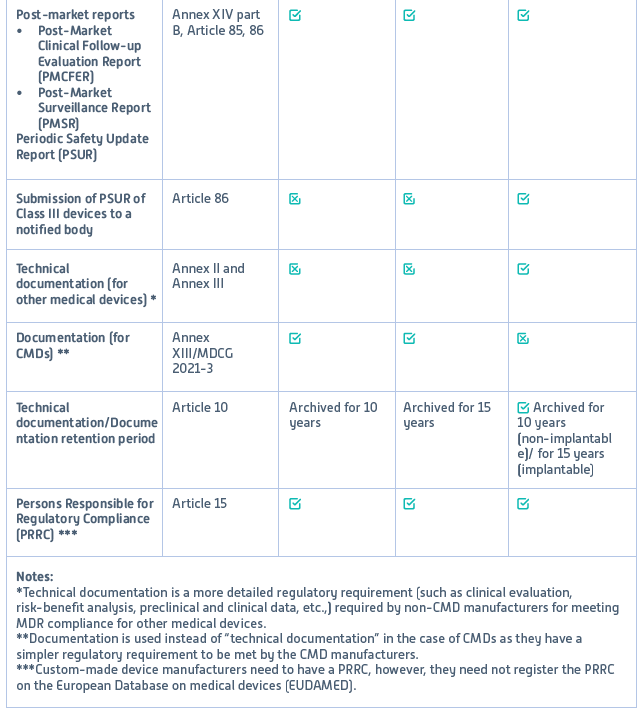
Reference: MDR Regulation (EU) 2017/745: Annex XIII (completely dedicated to custom-made devices); Annex IX: Conformity assessment based on a Quality Management System and assessment of technical documentation; Annex XI: Conformity assessment based on product conformity verification
What is the Conformity Assessment Procedure for Custom-made devices?
As per EU MDR requirements, all medical devices should comply with relevant regulatory requirements to ensure conformity. Figure 2 below describes the procedure for the conformity assessment of CMDs (such as notified body review) and compliance. The manufacturer must prepare a conformity assessment statement (as detailed in step 1, Figure 2) and keep up to date the documentation as stated in Annex XIII of MDR, including details such as manufacturing information, design, and performance of the device.
The manufacturers of CMDs need to follow instructions for use when using CE-marked parts, components, or materials. In case of change in any physical, chemical, or biological characteristics of these parts during the manufacturing of the CMDs, compliance of the finished product to the general safety and performance requirements of MDR Annex I needs to be met. Custom-made devices are classified using a similar risk-based approach that includes invasiveness, intended use, and duration of use as other medical devices. Manufacturers of CMDs must provide a classification reason to the notified body and details on why the device is classified as a CMD.
Figure 2: Procedure for Custom-made devices
MDR Compliance Timeline Extension
Unlike MDD regulations, MDR mandates the involvement of a notified body in the conformity assessment of Class III custom-made implantable devices. As a benefit of the extension for the manufacturers, it is permissible to put Class III custom-made implantable devices into service or place them on the market until 26 May 2026 without needing certification from a notified body. However, for these devices, the manufacturer or their EU-authorized representative must submit a formal application for conformity assessment to the notified body by 26 May 2024. Moreover, a written agreement with the notified body must also be signed by 26 September 2024. All other classes of CMDs can be placed on the market after meeting all the applicable MDR requirements and drawing a statement as per Annex XIII of MDR.
Transitioning from MDD to MDR is an ongoing challenge for many manufacturers wanting to keep their existing medical devices available in the EU market. At ClinChoice, we take great pleasure in assisting you in fulfilling these regulatory requirements, including the preparation of clinical evaluation reports and other regulatory reports for your medical devices. We are here to support you with a team of proficient specialists to ensure you meet the MDR regulatory requirements for your CMDs and other medical devices without experiencing any hassle.
About Us
ClinChoice is a leading global Contract Research Organization (CRO) with over 3,700 clinical research professionals across North America, Asia, and Europe. For more than 27 years, ClinChoice has been providing high-quality contract research services to pharmaceutical, biotechnology, medical device, and consumer products clients, encompassing a broad range of services and therapeutic areas. ClinChoice offers cutting-edge, full-service solutions for Clinical Trials, Regulatory Affairs, Medical Device Safety, Toxicology, and Medical Affairs.