The FDA’s Accelerated Approval (AA) pathway, introduced in 1992 amid the HIV/AIDS crisis, has played a pivotal role in expediting access to critical therapies, especially in oncology and rare diseases. However, concerns over delayed confirmatory trials and the extended availability of unverified treatments have prompted regulatory refinement.
In its new draft guidance issued in Jan 2025, the FDA now requires that confirmatory trials be underway at the time of approval. This fundamental shift enhances patient protection, reinforces sponsor accountability, and ensures that only rigorously validated therapies reach the market—thereby strengthening regulatory integrity and preserving public trust.
Key guidance updates: decoding the details
-
- Confirmatory Trials as a Prerequisite: Post-approval confirmatory trials must now be initiated before or within a defined timeframe following approval. Sponsors must ensure that patient enrolment has commenced, a comprehensive protocol is in place, and clearly defined timelines with measurable milestones are established for study execution and completion. This proactive approach minimizes exposure to unproven treatments, reinforcing accountability and prioritizing patient safety.
- Clearer Expectations for Endpoints: The guidance clarifies acceptable surrogate or intermediate clinical endpoints. Sponsors must present strong scientific rationale—expert opinions, data, and drug mechanism insights—to support predictive value. A deep understanding of the endpoint’s role in disease pathophysiology is essential in rare diseases, where correlation is challenging.
- Stricter Timelines and Reporting: The FDA now mandates enrolment targets, completion milestones, and biannual progress reports for confirmatory trials. Heightened scrutiny demands precise planning and execution to meet regulatory expectations.
- Expedited Withdrawal: Reinforcing Accountability: The FDA streamlines AA withdrawal if confirmatory trials fail, sponsors neglect obligations, or safety concerns arise, ensuring swift action on ineffective treatments.
- Early Engagement: Aligning Development with Expectations: Proactive consultations on pathways, endpoints, and trial design help sponsors meet FDA standards and potentially accelerate approvals.
Impact on the pharmaceutical industry
The FDA’s enhanced AA pathway imposes stricter requirements on drug development, demanding early confirmatory trials, innovative trial designs, and swift patient enrolment to prevent delays or revocation. Sponsors are now required to submit progress reports to the FDA on the status of these trials approximately every 180 days while the FDA can set specific post-approval study conditions, including enrolment targets and timelines. These changes increase financial commitments, intensify oversight, and demand greater accountability, compelling sponsors to adopt a more strategic, compliant, and proactive approach throughout development and post-marketing.
Executing with precision: strategies for rare disease trials under accelerated approval (aa)
Successfully executing accelerated trials for rare diseases demands precision, foresight, and adaptability.
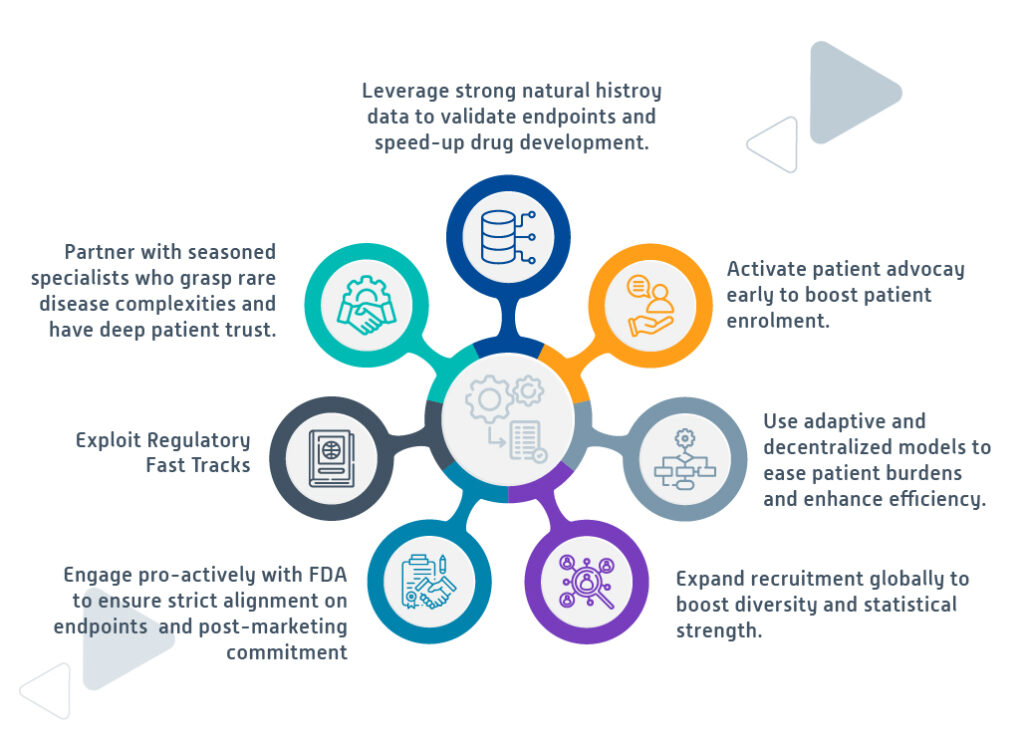
In conclusion, while challenging, AA for rare disease therapies is achievable through strategic planning, robust data, and innovative, globally focused trial designs.
From regulatory planning to meeting the endpoint – trust ClinChoice to lead the way
At ClinChoice, we help clients navigate the complexities of AA, addressing challenges such as intricate trial designs, resource constraints, and stringent regulatory requirements. Our expert team provides tailored, end-to-end solutions, driving efficiency from early-stage planning through regulatory submission and post-marketing commitments.
With extensive experience in rare disease trials, we deliver strategic, cost-effective approaches that ensure robust trial execution, optimize FDA interactions, and leverage regulatory incentives to expedite approval.
Partner with ClinChoice for a results-focused, streamlined path to approval, ensuring your therapy reaches the market faster and more effectively. With expertise, efficiency, and unwavering commitment, we turn innovation into impact.
